When r, p and M represent rate of diffusion, pressure and molecular mass, respectively, then the ratio of the rates of diffusion (rA/rB) - Sarthaks eConnect | Largest Online Education Community
Can the rate of effusion or diffusion be negative, in accordance with Graham's law? If so, how? - Quora
Rate of diffusion of a saturated hydrocarbon is about 1/6 th of that of hydrogen under similar conditions of temperature and pressure. What is the molecular formula of that hydrocarbon?



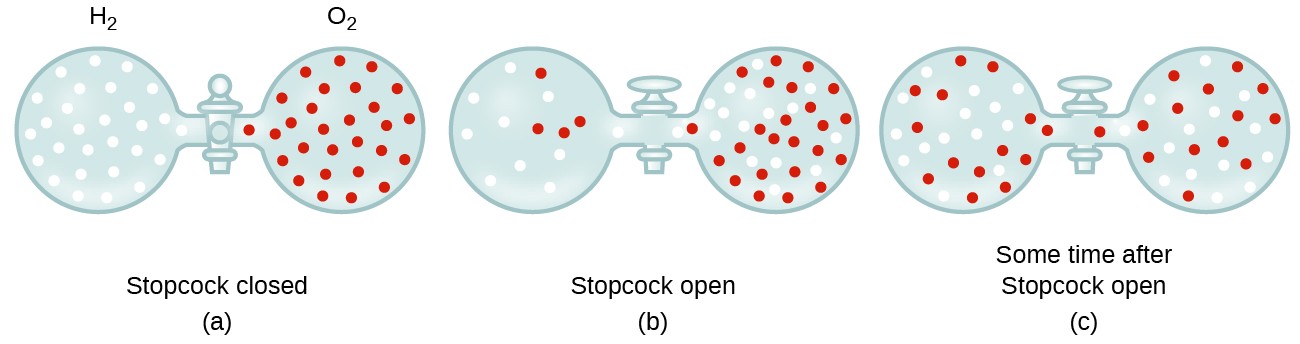






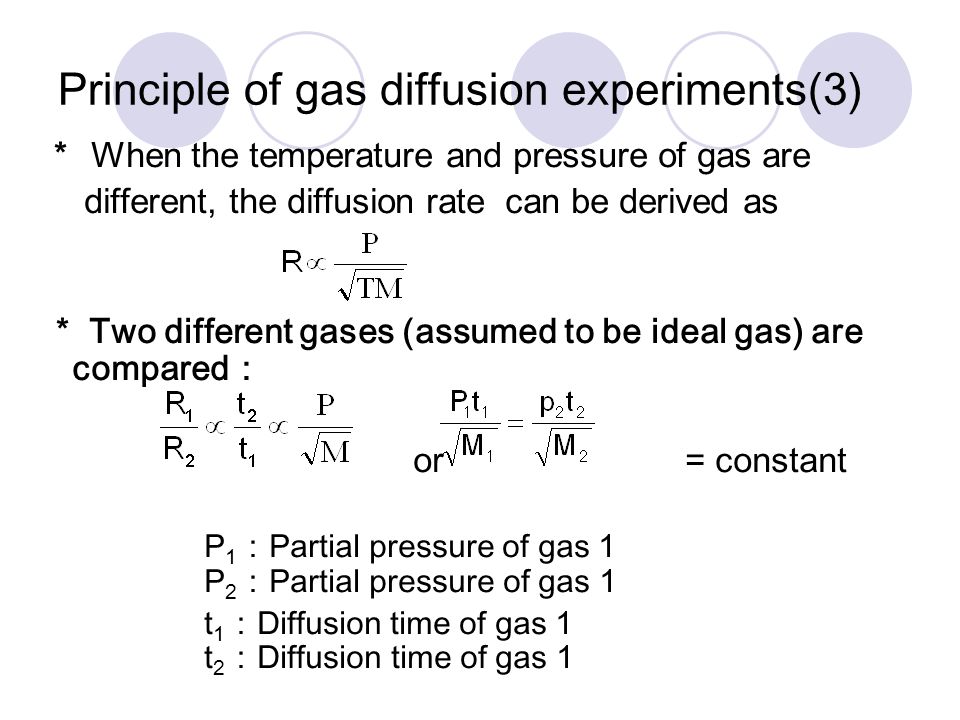

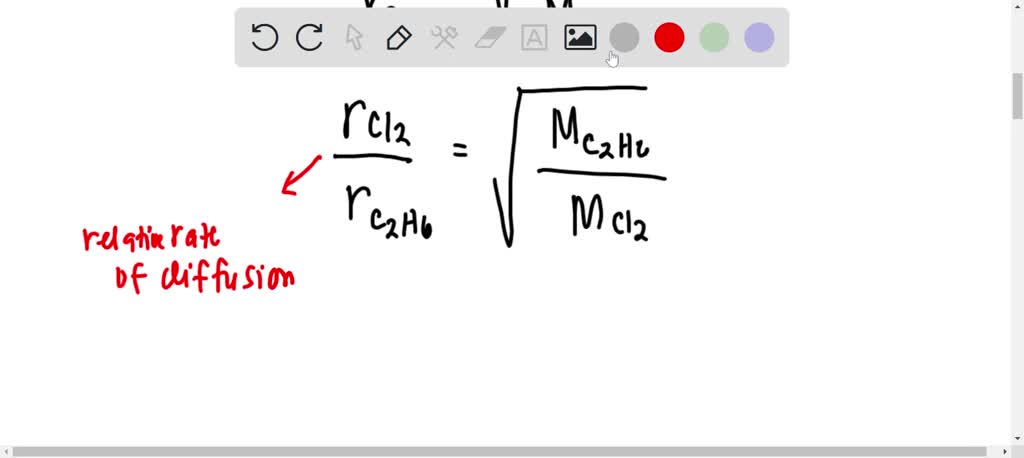


![ANSWERED] SolveLancer Test The rate of diffusion of two gases X Y are - Kunduz ANSWERED] SolveLancer Test The rate of diffusion of two gases X Y are - Kunduz](https://media.kunduz.com/media/sug-question-candidate/20210625230951982656-1884508.jpg)



