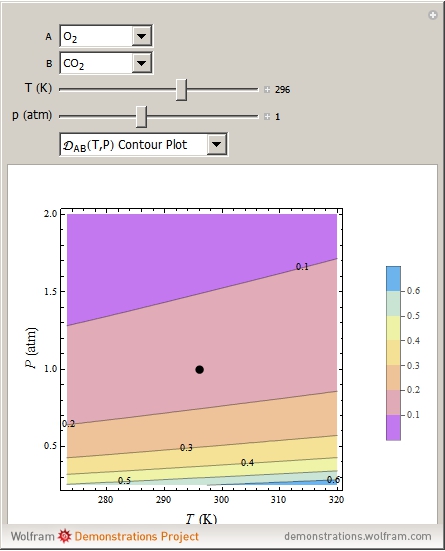
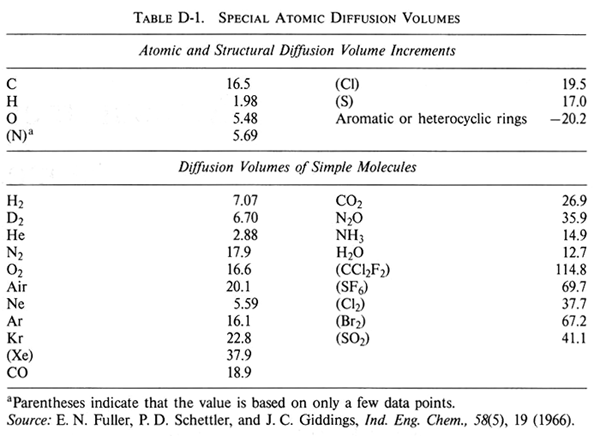
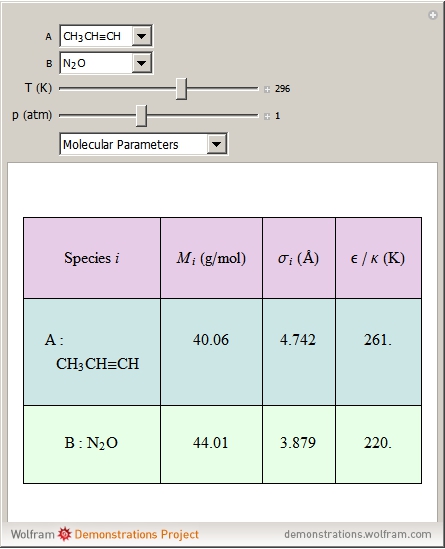
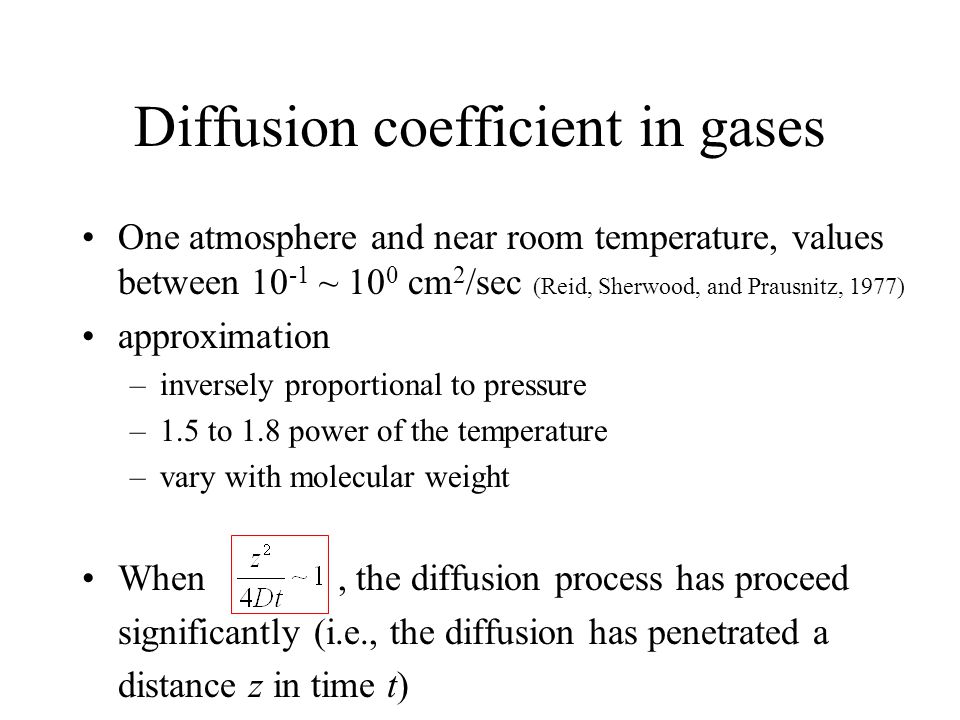
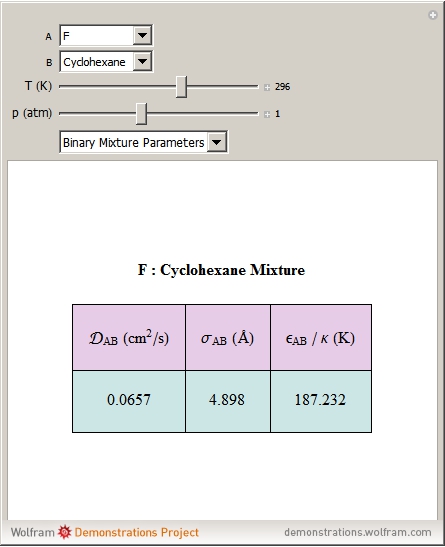
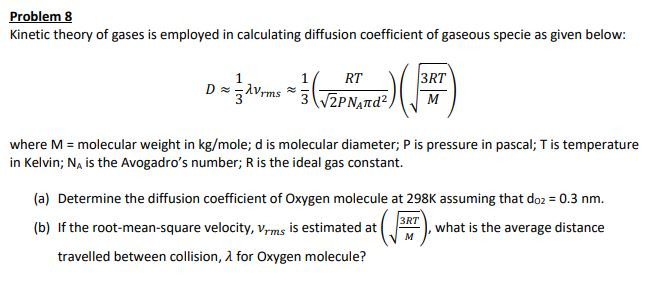
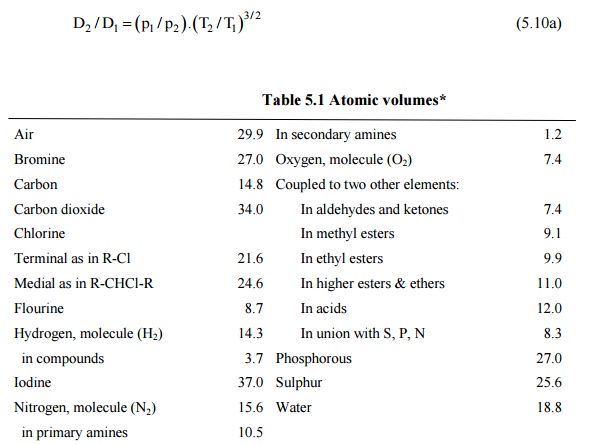
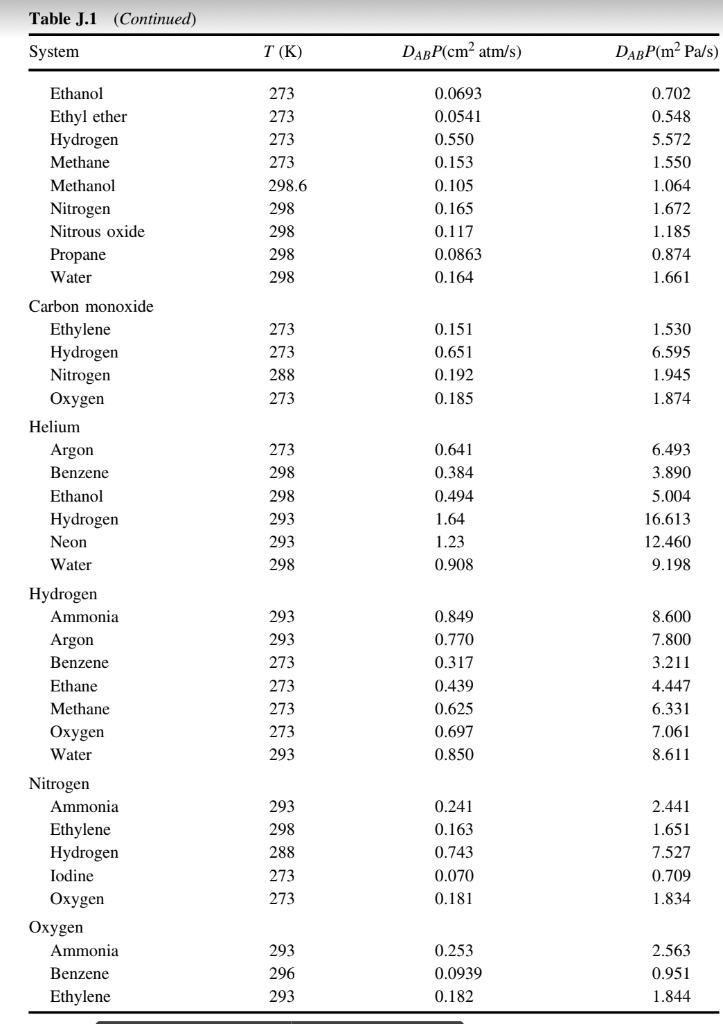
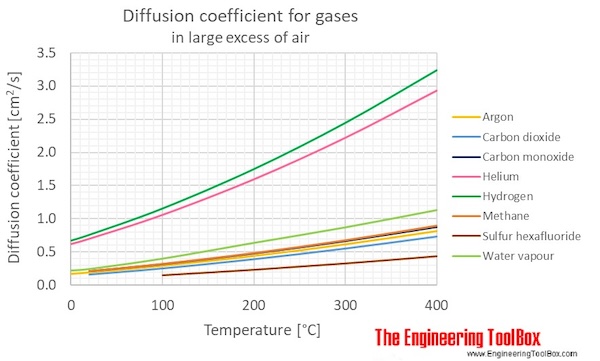
Molecular diffusion coefficients of several gases, ions and molecules.... | Download Scientific Diagram
![PDF] Self-Diffusion Coefficients of Lennard-Jones Liquids and Gases for Various Models in the Modified Free Volume Theory:Tables | Semantic Scholar PDF] Self-Diffusion Coefficients of Lennard-Jones Liquids and Gases for Various Models in the Modified Free Volume Theory:Tables | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/f5cfa3309b95ccd3f1ea1910385f7eac8bbb212f/5-Table1-1.png)
PDF] Self-Diffusion Coefficients of Lennard-Jones Liquids and Gases for Various Models in the Modified Free Volume Theory:Tables | Semantic Scholar

Diffusion Coefficients of CO2 and N2 in Water at Temperatures between 298.15 K and 423.15 K at Pressures up to 45 MPa | Journal of Chemical & Engineering Data

The diffusion coefficient of an ideal gas is proportional to its mean path and mean speed. The absolute temperature of an ideal gas is increased 4 times and its pressure is increased
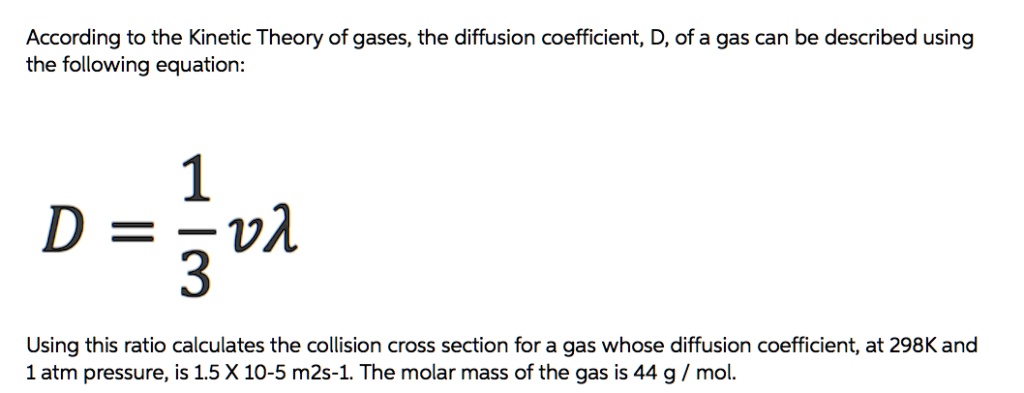
SOLVED: According to the Kinetic Theory of gases, the diffusion coefficient; D, of a gas can be described using the following equation: 1 D = v^ 3 Using this ratio calculates the
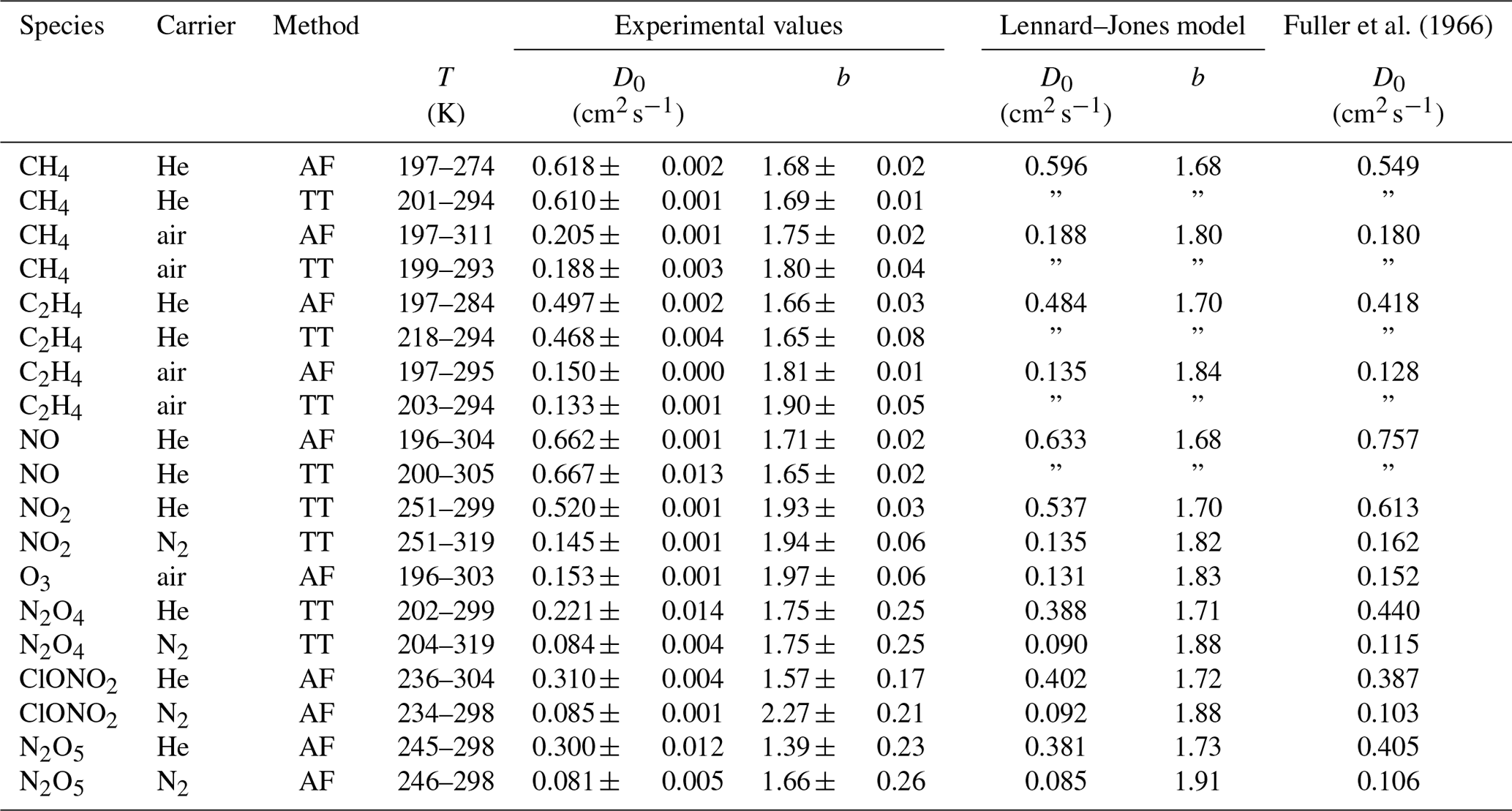
ACP - Technical note: Determination of binary gas-phase diffusion coefficients of unstable and adsorbing atmospheric trace gases at low temperature – arrested flow and twin tube method

Molecules | Free Full-Text | Difference Analysis of Gas Molecules Diffusion Behavior in Natural Ester and Mineral Oil Based on Molecular Dynamic Simulation
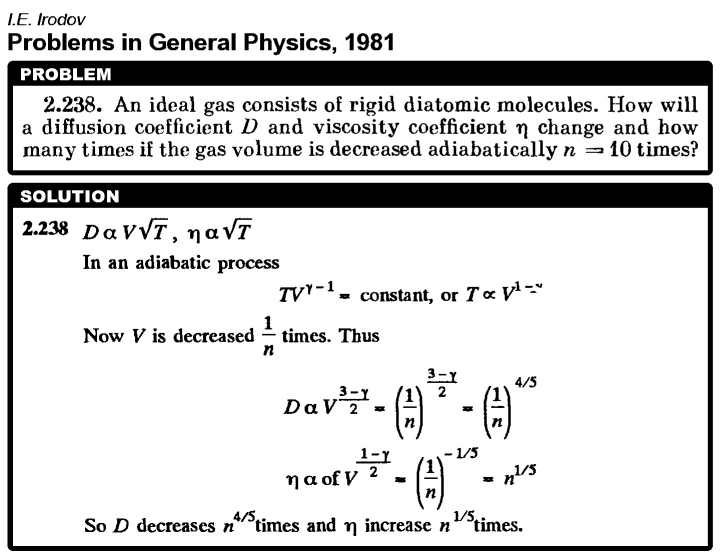
An ideal gas consists of rigid diatomic molecules. How will a diffusion coefficient D and viscosity coefficient n change and how many times if the gas volume is decreased adiabatic